Hello, everyone. My name is Daichi, an expert providing the information on the radiation issues in an easy-to-understand manner.
Today, I would like to respond to the following questions:
– What is the difference for approach for ionization, mass and charge, between alpha, beta and gamma ray?
– How different is penetration power and energy between the three kinds of radiation (this article especially focuses on alpha ray)?
Table of contents of this article
- Kinds of radiation and their characteristics (Vol. 2)
- Difference of characteristics between alpha, beta and gamma ray
- Approach for ionization
- Mass and charge
- Penetration power and energy of alpha ray
- Summary
I have been involved with the radiation-relevant issues, like the policy on the decontamination activities and the management of the Interim Storage Facility, after the accident of the Fukushima Daiichi Nuclear Power Plant in 2011.
I received a doctorate in the field of radiation, while working in Fukushima.
Kinds of radiation and their characteristics (Vol. 2)
In the previous article, kinds of radiation were briefly reviewed, and among a lot of kinds of radiation, three kinds of radiation: alpha, beta and gamma ray were explained, which need to be cared about, when addressing environmental contamination in the aftermath of unexpected events like an accident of a nuclear power plant, which are mainly covered in this blog.
Difference of characteristics between alpha, beta and gamma ray
Classification of alpha, beta and gamma ray (particle beam or electromagnetic wave) and and their structures are covered in the previous article, but other characteristics are also totally different between three kinds of radiation.
Specific examples for the differences are covered in the following sections.
Approach for ionization

As elaborated in this article, to make a long story short, ‘ionization’ is a phenomena, that orbital electrons in an atom core are pushed outward.
Ionization effects caused by ionizing radiation (radiation with ability to cause ionization effects) are phenomenon which could cause negative effects on human health, but the mechanisms which cause the ionization effects can be roughly classified into 2 categories.
Direct ionization
Direct ionization means, as its name implies, a phenomena in which ionizing radiation directly pushes outward orbital electrons.
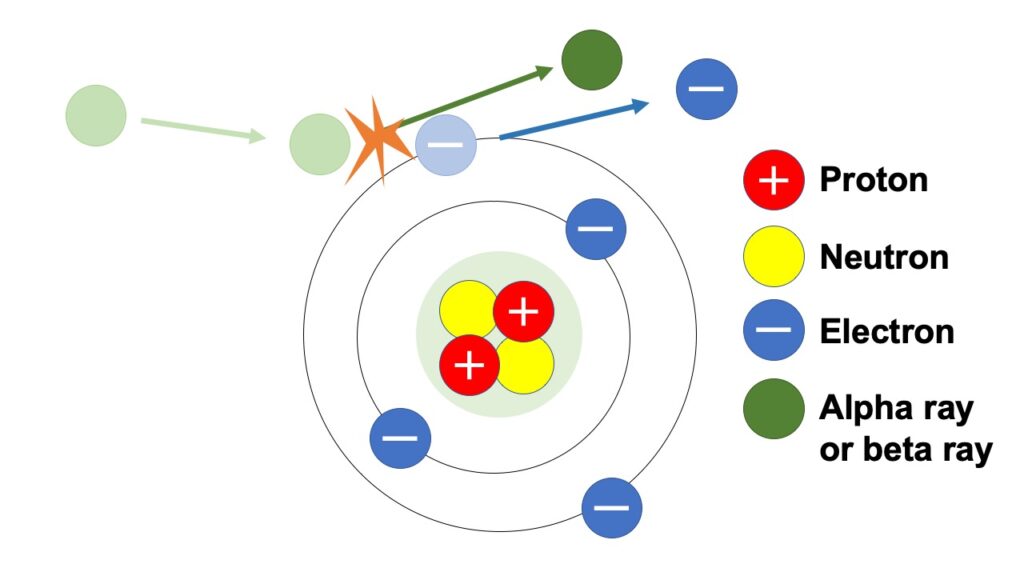
Of alpha, beta and gamma rays, which are focused on in the series of articles, alpha and beta rays, push outward orbital electrons directly, as shown in the above figure.
Indirect ionization
Indirect ionization means, as its name also implies, a phenomena in which ionizing radiation doesn’t ionize directly, but instead ionizes with secondary electrons, which are generated through interaction with other substances.
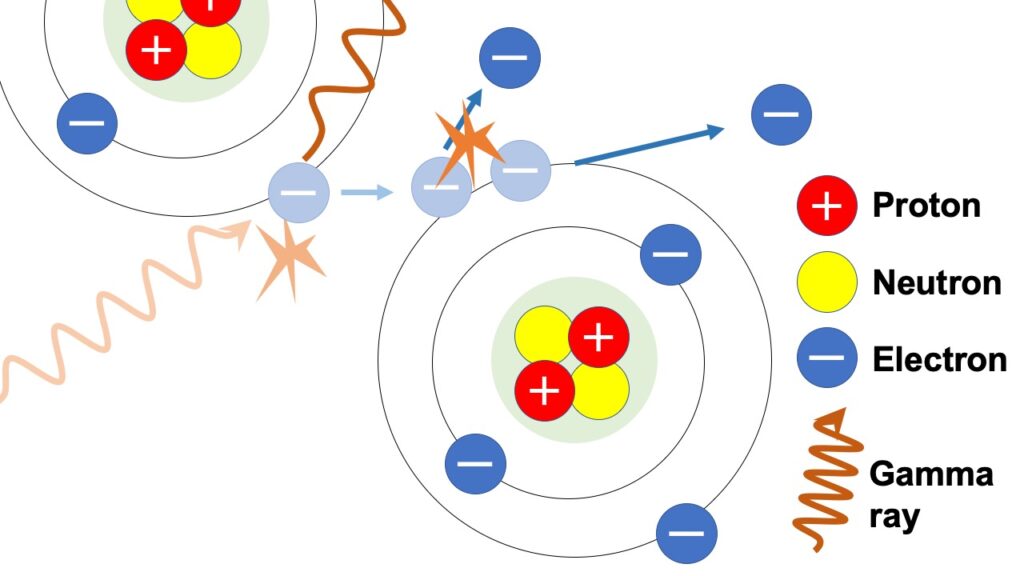
Of alpha, beta and gamma rays, gamma ray, which doesn’t have any charge, pushes outward orbital electrons through indirect ionization through interaction with other substances, as shown in the above figure.
Mass and charge

The following table just briefly summarizes the difference of mass and charge of alpha particle (ray), beta particle (ray) and gamma ray.
First, provided that mass of a beta particle is 1, mass of an alpha particle, which consists of 2 protons and 2 neutrons, will amounts to 7,000.
For the next, as explained in the previous article, because a beta particle is actually a positron or an electron, charge of a beta particle is, 1 (positron), and -1 (electron), respectively.
Charge of an alpha particle is 2, because it has 2 protons.
And at last, gamma ray has neither mass nor charge, because it is electromagnetic wave.
| Alpha particle (ray) | Beta particle (ray) | Gamma ray | |
|---|---|---|---|
| Mass | 7,000 | 1 | N.A. |
| Charge | 2 | 1 or -1 | N.A. |
Penetration power and energy of alpha ray

Taking account of the aforementioned difference for approach for ionization, mass as well as charge of each kind of radiation, penetration power of each kind of radiation will be covered in this section.
This article especially focuses on the penetration power of alpha ray.
As shown in the table above, an alpha particle has much larger mass than a beta particle, although they are both charged particles.
Therefore, it travels slower, compared with a beta particle with almost same amount of energy.
In addition, an alpha ray has larger charge than a beta particle, so once it is released, it travels straight by reacting with other nearby atom cores and electrons sequentially.
But it reacts with a lot of atoms in the vicinity of release point, and it quickly loses its energy and can’t travel so long distance.
One important thing which needs to be known regarding alpha particles, is that each alpha particle (ray) has its own unique energy, and it depends on kinds of radionuclides, which emit alpha particles.
Specifically, each alpha-radionuclide emits alpha particle(s) with one line spectrum or multiple line spectra.
As specific examples of radionuclides, Thorium 232, Radium 226 and Americium 241 can be taken here, and they have distribution patterns with a single line spectrum, or multiple line spectra within energy bands of 3.8-4.0 (MeV), 4.6-4.8 (MeV) and 5.4−5.5 (MeV), respectively.
You can see the energy bands of each kind of alpha particle, as well as their main puroposes for application in the following table.
| Main energy of alpha ray (MeV) | Main purpose for application (Ex.) | |
|---|---|---|
| Thorium 232 | 3.8 - 4.0 | (Natural Occurring Radioactive Material) |
| Radium 226 | 4.6 - 4.8 | Luminous paint for dial faces of watches |
| Americium 241 | 5.4 − 5.5 | Thickness meters, smoke detector |
Summary
Today, regarding the 3 kinds of radiation (alpha, beta and gamma rays), the difference of approach for ionization, mass as well as charge are covered, and based on the difference, penetration power, especially focusing on alpha ray and its energy are elaborated.
By the way, abovementioned contents are summarized in the following videos.
It would be appreciated to visit them at your convenience.
– Japanese version
– English version
You can read the same article in Japanese here.
Thank you very much for reading this article.
See you next time!


コメント