Hello, everyone. My name is Daichi, an expert providing the information about the radiation issues in an easy-to-understand manner.
In this article, regarding the impact from natural radiation, the magnitude of whole impact in Japan and in the world, and in particular, especially impact caused by radiation coming from outer space to the Earth and emitted from the earth were elaborated.
This time, the rest of the two impacts, that is, the impact from breathing and from foods will be elaborated.
In other words, this article responds to the following questions:
– What is the impact from natural radiation caused by breathing?
– What is the impact from natural radiation caused by eating foods?
Table of contents of this article
- (Impact from breathing and food is also covered) What is natural radiation? (Vol. 2)
- Impact from natural radiation caused by breathing
- Impact from natural radiation caused by eating foods
- Pb-210、Po-210
- K-40
- C-14
- Tritium(H-3)
- Summary
I have been involved with the radiation-relevant issues, like the policy on the decontamination activities and the management of the Interim Storage Facility, after the accident of the Fukushima Daiichi Nuclear Power Plant in 2011.
I received a doctorate in the field of radiation, while working in Fukushima.
(Impact from breathing and food is also covered) What is natural radiation? (Vol. 2)
Now let me elaborate in sequence the impacts from natural radiation caused by breathing and foods.
Impact from natural radiation caused by breathing
As covered in this article, radioactive materials, by which we are impacted in our daily life through breathing, are radon and thoron.
Here radon refers to radon with a mass number of 222, and thoron refers to radon with a mass number of 220.
Both of them are radioisotopes of radon, but sometimes they are still called with different names, as a remnant of time, when they are thought as different elements (For more information about mass number and radioisotope, please visit this article).
As slightly touched upon in this article, radon is produced through alpha decay of radium-226 in the ground, and thoron is produced through alpha decay of radium-224 in the ground.
Both of them are in a gaseous state at room temperature, therefore they exist in the air around us, and we intake them on a daily basis through breathing.
The radioactivity concentration varies regions to regions, but when viewed globally, for example, it tends to be higher in Northern Europe, than other regions, due to geological reasons (e.g. the amount of radium in soil), and to construction materials (e.g. stone, woods) (it depends on the kinds of stones, but for example, granite contains a lot of uranium and thorium).
And both radon and thoron are significantly heavier than air (average molecular weight: 29), and they tend to remain indoors like underground, therefore, for example, measures like ventilation is sometimes needed in places, where concentrations of radon and thoron are high.
By the way, people are exposed to radiation emitted by radioisotopes like lead and polonium by smoking, although its amount is quite small.
Therefore, it would be recommended to stop smoking, if possible.
Impact from natural radiation caused by eating foods

First of all, a mechanism will be briefly explained, in which natural radioactive materials are contained in foods.
As explained in this article, radioactive materials are contained in the earth, and sometimes plants absorb the radioactive materials from their roots.
Regarding animals, within the food chain of terrestrial and marine ecosystems, the radioactive materials are taken by predators and sometimes they are concentrated.
By taking foods which absorb radioactive materials during these processes, we take radioactive materials usually in our body on a daily basis.
As covered in this article, dose derived from foods is around 0.99mSv per year in Japan, and its breakdown is as described in the following figure.
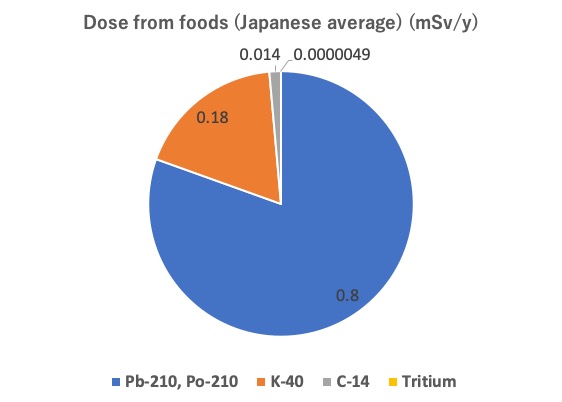
(Source: Created based on the UNSCEAR 2008 Report and the Radiation in the Living Environment (Calculation of Public Dose) 3rd Edition, Expanded Edition (2024) (The Nuclear Safety Research Association)
– Pb-210, Po-210: 0.80mSv/y
– K-40: 0.18mSv/y
– C-14: 0.014mSv/y
– Tritium(H-3): 0.0000049mSv/y
Let me add some more explanation for each radionuclide.
Pb-210、Po-210
Both Pb-210 and Po-210 are radionuclides that belong to the Uranium series (Radium series) produced in the process of alpha decay of radon-222, which was explained just earlier.
Unlike radon-222, they exist as solid materials at room temperature, and they deposit on the surface of the ground, precipitate in rivers and the ocean, and absorbed by creatures like fish.
Further to that, they are absorbed in the food chain in the terrestrial and marine environment, and taken into our body through our diet on a daily basis.
However, a dose of 0.80mSv per year for Japanese people is not the amount that causes adverse health effects, so, in terms of radiation protection, I think it is not necessary to worry about eating them too much (but be careful in terms of nutrition balance, because it is not good to have unbalanced diet).
K-40
Potassium is one of the essential elements that makes up human tissues, along with carbon, oxygen, hydrogen and nitrogen.
Of potassium in the nature world, around 0.01% of potassium is potassium-40 (K-40), a radioisotope of potassium, and I could say that it is included almost all of foods.
However, radioactivity concentration varies greatly from food to food as follows:
– Rice: 30Bq/kg
– Milk: 50Bq/kg
– Beef: 100Bq/kg
– Fish: 100Bq/kg
– Dry milk: 200Bq/kg
– Spinach: 200Bq/kg
– Potato chips: 400Bq/kg
– Green tea:600Bq/kg
– Dried shiitake mushroom: 700Bq/kg
– Dried kelp: 2,000Bq/kg
I think that the following are reasons why the radioactivity concentration of dried kelp is especially high.
– Seaweed like kelp has a long growth period.
– Kelp has a characteristics, easily to absorb radioactive materials.
– Radioactivity concentration per kilogram increases, as a result of decrease of moisture, caused by drying kelp.
However, concentration of potassium in human body is maintained at a constant level, so I think that it will not cause any issues, from the perspective of exposure to radiation, even if you eat so much kelp in your daily life (as mentioned in the section on Pb-210 and Po-210, you need to be careful in terms of nutritional balance).
C-14
C-14 is produced through collision of cosmic rays and nitrogen atoms in the air.
Its proportion in the whole carbon atoms is quite small, but C-14 is captured in the CO2 in the air, and further absorbed in flora and fauna, through breathing and photosynthesis.
As long as flora and fauna are alive, the ratio of C-12 and C-14 remains constant, but after they die, C-14 is not taken anymore, and the ratio decreases gradually due to radioactive decay (physical half-life: 5,730 years).
As mentioned in this article, the method to estimate the time when the flora and fauna lived, taking advantage of the changes of the ratio, is radiocarbon dating.
Tritium(H-3)
As explained in this article, tritium is a radioisotope of hydrogen and most abundant radioactive material in the treated water released from Fukushima Daiichi NPS.
In the nature world, as is the case with carbon-14 covered above, it is produced as a result of collision of cosmic ray and nitrogen atoms in the atmosphere.
Most of the generated tritium are incorporated into water molecules and exist as liquid, but proportion of tritium in the whole hydrogen atom is quite small: less than around from one trillionth to one hundred trillionth.
The tritium that exists as liquid is taken into our body through diet (drinking water) and influences us, although we don’t have to worry about adverse effect on our health, because the amount is much much less than other radionuclides, as mentioned above.
Summary
As a continuous work from the previous, related with natural radiation, impact caused by breathing and diet was in detail elaborated.
Speaking of radiation, it attracts attention in the special occasions like accident of nuclear power plants and treatment in hospitals, but it exists around us.
Therefore, if you learn its dose, I think tha you can understand better about radiation, by comparing with dose in such special occasions.
By the way, above-mentioned contents are summarized in the following videos.
– Japanese version
– English version
You can read the same article in Japanese here.
Thank you very much for reading this article.
See you next time!



コメント