Hello, everyone. My name is Daichi, an expert providing the information on the radiation issues in an easy-to-understand manner.
Today, I would like to respond to the following questions:
– What is the isotope/radioisotope?
– What are the specific examples of isotope/radioisotope?
Table of contents of this article
- (Similar but different) What is the radioisotope?
- What is the isotope?
- What is the radioisotope?
- Examples of (radio)isotope
- Hydrogen
- Carbon
- Summary
I have been involved with the radiation-relevant issues, like the policy on the decontamination activities and the management of the Interim Storage Facility, after the accident of the Fukushima Daiichi Nuclear Power Plant in 2011.
I received a doctorate in the field of radiation, while working in Fukushima.
(Similar but different) What is the radioisotope?
To make a long story short, the radioisotope is the isotope with radioactivity.
Then what is the isotope?
Let’s go into detail in the following sections.
What is the isotope?

The isotope is the relationship between elements with the same number of proton, but the different number of neutron, or it refers the each nuclide.
Therefore, it can be said, that ‘A is an isotope of B’ as well as ‘A and B are under the relationship of isotope for each other’.
As explained in this article, the number of proton determines the kind of element, and the proton number plus the neutron number equals the mass number.
Therefore, for example, it can be rephrased, that the isotope is ‘the relationship between the same elements with the different mass number, or it refers the each nuclide’.
By the way, the proton number and the mass number are sometimes described as the following figure, to understand the situation of an element at first sight.
As explained in this article, this is an example of a helium atom and on the top-left the mass number (= the proton number plus the neutron number) is written, and on the bottom-left the atom number (= the proton number) is written.
And the neutron number can be calculated by subtracting the atom number from the mass number (in this case: 4-2=2).
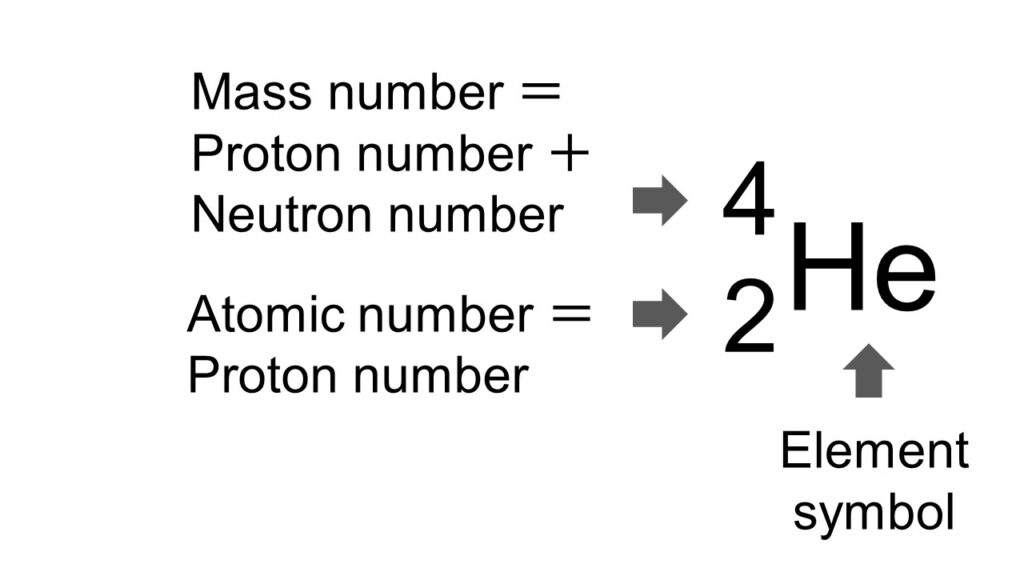
The specific kinds of isotopes will be elaborated in the following sections.
What is the radioisotope?
As aforementioned, the radioisotope is the isotope with radioactivity (Regarding the radioactivity, please visit this article).
The isotope is, as also already mentioned, for example, is a nuclide with the same proton number, but with the different neutron number.
There are some isotopes emitting radiation after the radioactive decay due to its instability, by having the different neutron number, and they are called radioisotope.
Examples of (radio)isotope
Then let’s look at specific examples of isotopes and radioisotopes.
Hydrogen

Hydrogen is a representative example of isotope and radioisotope.
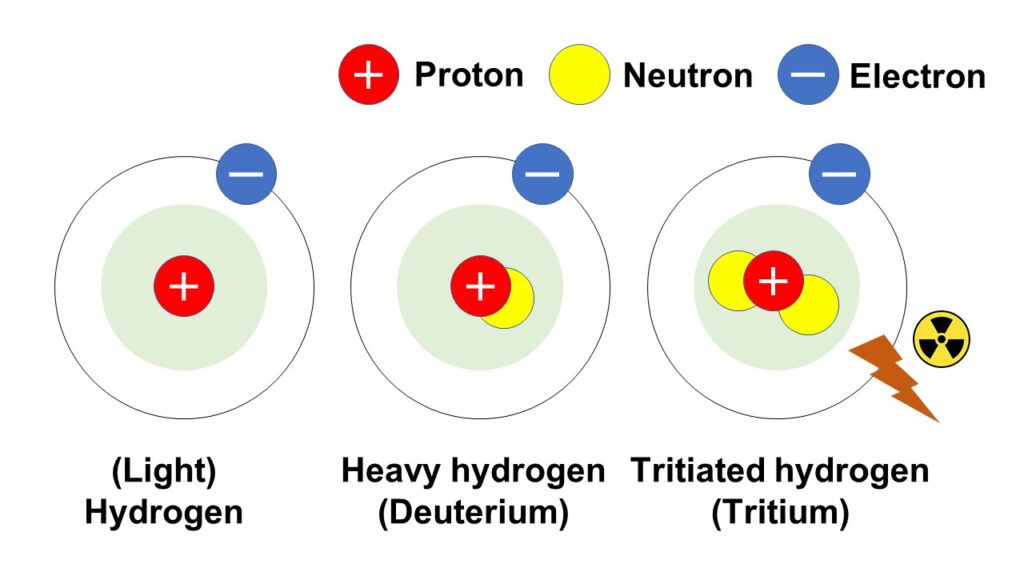
As shown in the below figure, the proton number of hydrogen is 1, and among hydrogen isotopes, a hydrogen atom without neutron (sometimes called the ‘light hydrogen’ to distinguish with the ‘heavy hydrogen’ in the below sentences) exists in the environment dominantly with the highest existence proportion.
And there is another isotope called ‘heavy hydrogen’ (deuterium) with one neutron, in other word, with the mass number of 2.
The deuterium is used as a moderating material for neutron in the nucleus reaction, and it is expected to be used as fuel for nuclear fusion power generation.
In addition, there is another isotope with 2 neutrons, in other words, with the mass number of 3, called tritiated hydrogen, or tritium, and the tritiated hydrogen is a radioisotope with radioactivity.
It exists in nature slightly with quite small proportion, but many of you might know as a radioactive material, which are released into the environment in a planned manner, from the nuclear industry-relevant facilities, such as nuclear power plants.
There are other isotopes for hydrogen i.e.: from the hydrogen-4 (mass number: 4) to the hydrogen-8 (mass number: 8), but they can be obtained with artificial synthesis and their physical half-lives are very short, so details for these isotopes are here cut.
Carbon

There are a number of examples for isotope, but here isotopes of carbon are covered.
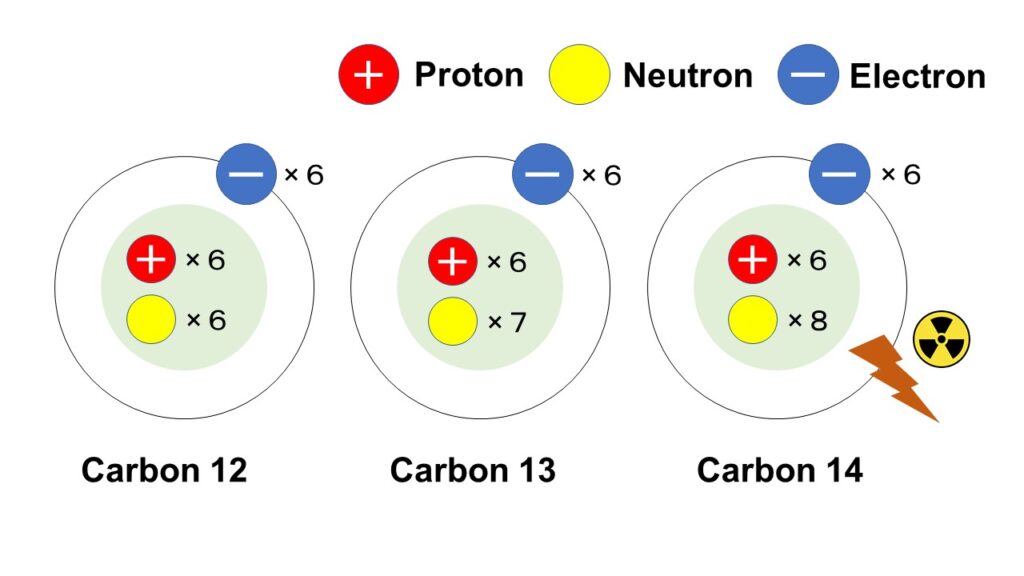
There are a lot of isotopes for carbon as hydrogen: from ‘carbon 8 (mass number is 8)’ to ‘carbon 22 (mass number is 22)’, but three representative examples: ‘carbon 12’, ‘carbon 13’ and ‘carbon 14’ are introduced in this section.
In the natural environment, most of the carbon exists as carbon 12, and very few carbon exists as carbon 13, and carbon 14 also exists with much less proportion compared with carbon 13.
Isotopes other than these three isotopes can be artificially synthesized, and their physical half-lives are quite short.
By the way, proton number of carbon is 6, therefore, neutron numbers of ‘carbon 12’, ‘carbon 13’ and ‘carbon 14’ are 6, 7 and 8 respectively, and carbon 14 is a radioisotope with radioactivity.
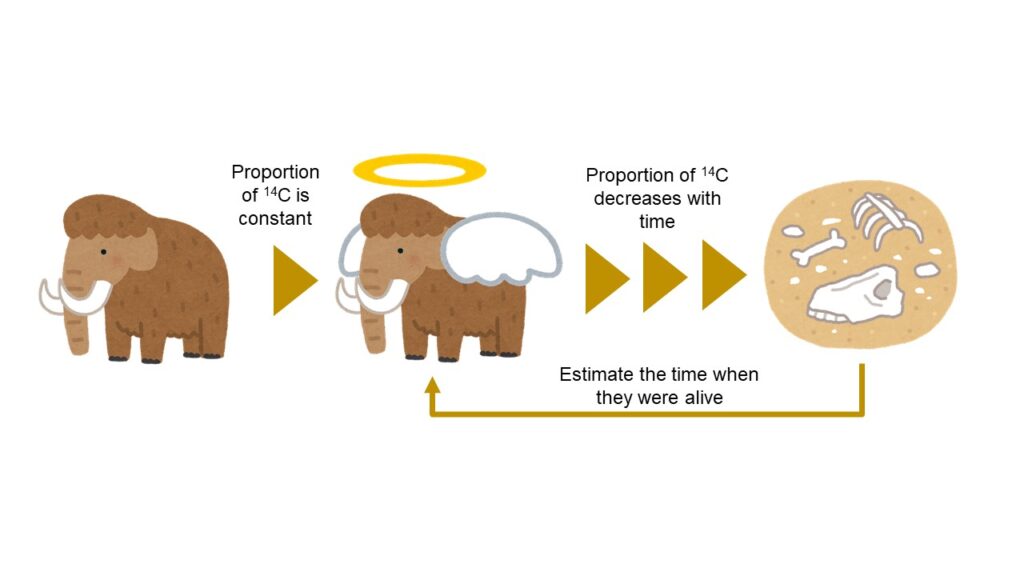
The carbon 14 is a radionuclide to be analyzed in order to estimate the period of times, when flora and fauna existed in the earth, by the radiocarbon dating methodology.
To make a long story very short, proportion of carbon 14 within the body of flora and fauna can be almost constant, by carbon provided through foods taken while they were alive.
After their death, however, existence proportion of carbon 14 would decrease because provision of carbon would cease.
In other words, by analyzing the present proportion ratio of carbon 14, the ages can be estimated when the flora and fauna existed on the earth.
This methodology is applied to various kinds of fields of study, like archaeology and art.
In the archaeology, it is used to estimate the ages when flora and fauna or cities found as ruins existed, and in the art study, it is used to estimate the ages when paintings were actually drawn, for example, by analyzing the plants used to make canvases.
Summary
This article covers isotope and radioisotope.
Isotope is the relationship between elements with the same number of proton, but the different number of neutron, or it refers the each nuclide, and radioisotope is isotope with radioactivity.
And as examples isotopes and radioisotopes of hydrogen and carbon are covered, and specific examples for application in the study fields of archaeology as well as art are provided.
By the way, the following videos are created, which explain the almost same contents above.
Please take a look at them at your convenience.
– Japanese version
In addition, please also visit the following video recapitulating the same contents within 3 minutes and 1 minute.
<3 minutes>
<1 minute>
– English version
Also for English version, please visit the following video recapitulating the same contents within 3 minutes and 1 minute.
<3 minutes>
<1 minute>
– German version
Only short version within 3 minutes and 1 minute, but the same contents were recapitulated in the following German video.
<3 minutes>
<1 minute>
– French version
Only short version within 1 minute, but the same contents were recapitulated in the following French video.
You can read the same article in Japanese here.
Thank you very much for reading this article.
See you next time!



コメント