Hello, everyone. My name is Daichi, an expert providing the information on the radiation issues in an easy-to-understand manner.
Today, I would like to respond to the following questions:
– What kind of radiation exist?
– What are specifically: Alpha ray, Beta ray and Gamma ray?
Table of contents of this article
- Kinds of radiation and their characteristics (Vol. 1)
- Kinds of radiation
- Alpha ray
- Beta ray
- Gamma ray
- Summary
I have been involved with the radiation-relevant issues, like the policy on the decontamination activities, after the accident of the Fukushima Daiichi Nuclear Power Plant in 2011.
I received a doctorate in the field of radiation, while working in Fukushima.
Kinds of radiation and their characteristics (Vol. 1)
In the previous article, kinds of radiation were briefly covered, but in this article, the kinds of radiation are focused on, which are targeted in this blog and which need to be cared about, especially after the unexpected discharge into the environment (e.g.: accidents of nuclear power plants), and their characteristics are elaborated.
Kinds of radiation
As explained in this article, radiation can be roughly categorized into ‘ionizing radiation’ and ‘non-ionizing radiation’.
Non-ionizing radiation is electromagnetic wave without ionizing effect (phenomena in which orbital electron(s) in an atom core is/are hit and push out of the atom.), and visible light, infrared ray, electric wave are examples of non-ionizing radiation.
Ionizing radiation is electromagnetic wave or particle beam with ionizing effect.
If people are exposed to too much ionizing radiation, negative effects on their body appear, like the deterministic effects or stochastic effects (please refer to this article).
In this article onward, as mentioned earlier, among the many kinds of ionizing radiation, three kinds of radiation (alpha ray, beta ray as well as gamma ray) are focused on, which especially need to be cared about, in the aftermath of the unexpected discharge of radioactive materials into the environment, like accidents of nuclear power plants, and their characteristics are elaborated.
Alpha ray

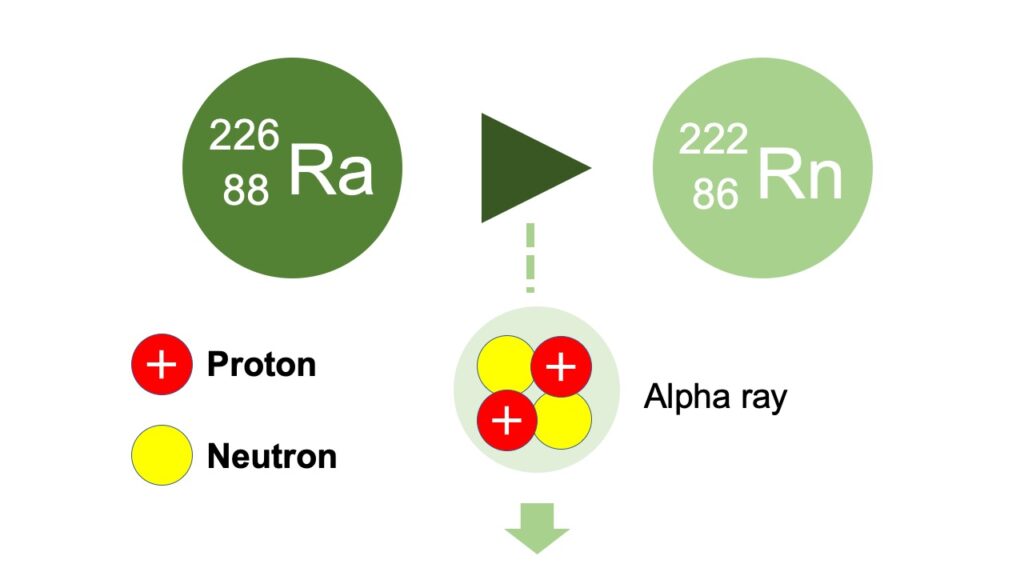
A particle, which is released as a result of alpha decay, and composed of 2 protons and 2 neutrons (= atom core of helium atom), is called alpha particle, and its flow is called alpha ray.
An alpha particle has 2 protons but no electrons, therefore it is a charged particle with positive charge.
By the way, if an alpha particle is released, the number of protons is reduced by 2 from the original atom, therefore the atomic number also decreases by 2 and it becomes a different kind of atom.
The number of neutron is also reduced by 2, so the mass number also decreases totally by 4, together with the reduction of 2 protons.
For example, if a radium 226 with the atomic number of 88, emits alpha ray as a result of alpha decay, it becomes radon 222 with the atomic number of 86.
An alpha particle excites the fluorescent materials, therefore, once small amounts of radium were used as luminous paint, at dial faces of watches, and ladies who painted radium on faces of watches in the factories suffered from health issues.
They have been, however, already replaced with something safer.
Reference:Wikipedia(Radium Girls)
Beta ray
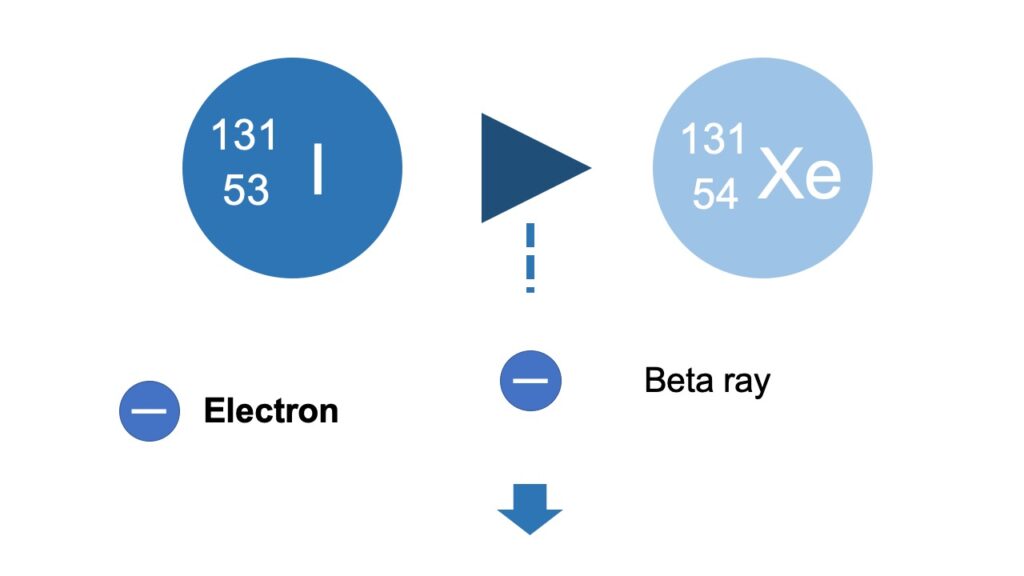
An electron or positron, which is emitted as a result of beta decay, is called beta particle, and its flow is called beta ray.
An electron has a negative charge, and if an electron is released, the atomic number increases by 1, because a neutron changes into a proton, by emitting an electron and the proton number increases by 1.
And a proton has a positive charge, and if a proton is released, the proton number decreases by 1, because a proton changes into a neutron, by emitting a positron and the proton number decreases by 1.
The mass number doesn’t change, because the sum number of protons and neutrons doesn’t change.
For example, if an iodine 131 with the atomic number of 53 emits a beta ray as a result of beta decay, as a form of an electron, it becomes xenon 131 with the atomic number of 54.
Iodine has a characteristic to tend to be accumulated in a thyroid, so beta ray emitted by iodine 131, is used to cure thyroid cancer, taking advantage of this characteristic.
Gamma ray

Electromagnetic wave emitted through gamma decay is called gamma ray.
Unlike alpha ray and beta ray, gamma ray doesn’t have any charge and it travels space with mutual and successive interaction of electric field and magnetic field.
Even if gamma ray is emitted, neither the proton number nor the neutron number changes, that is, neither the atomic number nor the mass number changes.
Cobalt 60 can be taken as an example of a radioactive material, which emits gamma ray.
After cobalt 60 with the atomic number of 27, changes into nickel 60, by emitting beta ray as a result of beta decay, the nickel 60 further emits excessive energy as gamma ray as a result of gamma decay.
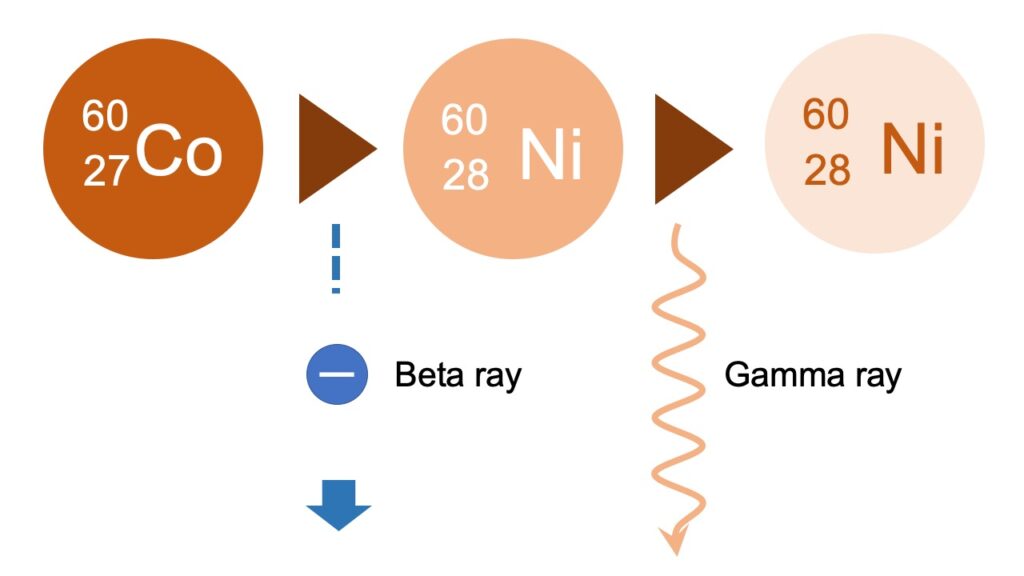
Taking advantage of its impact on living things, gamma ray emitted through the two-stage decay is, for example, used to extend shelf life of foods, by irradiating gamma ray as an alternative pesticide or medicament.
Summary
Among a lot of kinds of radiation, this article briefly covers the concept, characteristics as well as its specific use of three kinds of radiation: alpha, beta and gamma ray, which are especially needed to be cared about after unexpected events and following environmental pollution like accident of nuclear power plants.
By the way, abovementioned contents are summarized in the following videos.
It would be appreciated to visit them at your convenience.
– Japanese version
In addition, please also visit the following video recapitulating the same contents within 1 minute.
– English version
Also for English version, please visit the following video recapitulating the same contents within 1 minute.
– German version
Only short version within 1 minute, but the same contents were recapitulated in the following German video.
– French version
Only short version within 1 minute, but the same contents were recapitulated in the following French video.
You can read the same article in Japanese here.
Thank you very much for reading this article.
See you next time!


コメント